Zydus' Virafin gets emergency use approval for treating moderate COVID-19 cases

Zydus' Virafin gets emergency use approval for treating moderate COVID-19 cases
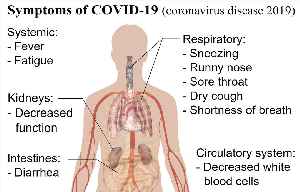
The Drugs Controller General of India (DGCI) has approved emergency use for Zydus Cadila's Pegylated Interferon alpha-2b, 'Virafin' for treating moderate COVID-19 infection in adults.
A release by Cadila Health said, "The drug has also shown efficacy against other viral infections."Speaking on the development, Dr Sharvil Patel, Managing Director, Cadila Healthcare Limited said: "The fact that we are able to offer a therapy which significantly reduces viral load when given early on can help in better disease management.
It comes at a much-needed time for patients and we will continue to provide them access to critical therapies in this battle against COVID-19." This government's approval for use of the antiviral drug comes at a time when India's daily COVID tally is crossing the 3-lakh mark.
India recorded 3,32,730 new COVID-19 cases in the last 24 hours, the highest single-day spike since the pandemic broke out last year.
India has crossed the mark of 3 lakh COVID-19 cases for two consecutive days now.
This has taken the cumulative count of the COVID infection in the country to 1,62,63,695.

![What is Virafin? How does the Zydus Cadila drug work? | Oneindia News [Video]](https://video.newsserve.net/300/v/20210424/1318965859-What-is-Virafin-How-does-the-Zydus-Cadila.jpg)
![Covid-19: Centre to fast-track emergency approval for vaccines| Oneindia News [Video]](https://video.newsserve.net/300/v/20210413/1318552520-Covid-19-Centre-to-fast-track-emergency-approval.jpg)
![Vaccinations for 12 to 15-year-olds could be available in weeks [Video]](https://video.newsserve.net/300/v/20210412/2104122323-Vaccinations-for-12-to-15-year-olds-could.jpg)