Hims and Hers to Offer Weight Loss Injections
Hims and Hers to Offer Weight Loss Injections
Hims and Hers , to Offer Weight Loss Injections.
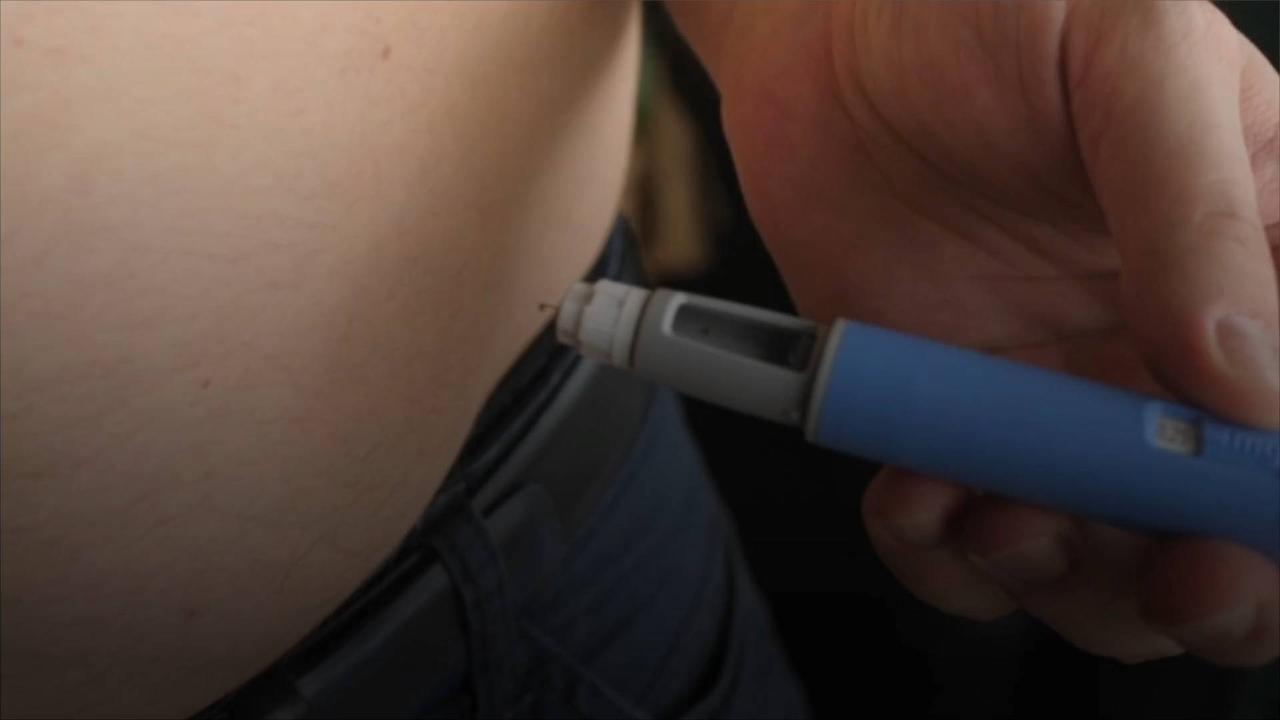
GLP-1 medications like Ozempic and Wegovy have become increasingly popular.
However, the market has faced some supply constraints recently because of that popularity.
On May 20, Hims & Hers Health announced that it will be providing access to compounded GLP-1 weight loss injections, CNBC reports.
.
Company shares jumped over 30% following the announcement.
Compounded versions of medications are created by certain manufacturers who meet FDA requirements when there are medication shortages.
However, the products are not tested for safety and efficacy by the FDA.
The new offering of compounded GLP-1 medications can be accessed by getting a prescription from a health care provider on the Hims & Hers site.
Branded GLP-1 injections will be made available once the supply becomes more consistent.
The price for compounded injections will start at $199 per month, CNBC reports.
.
Prior to announcing its GLP-1 offerings, Hims & Hers revealed in an earnings report... ... that it anticipates its weight loss program to garner over $100 million worth of revenue by December 2025.
Hims & Hers CEO Andrew Dudum said that he is "confident" that a steady supply of the compounded injections will be available for customers.
We have a certain degree of exclusivity with that facility that will guarantee our consumers consistent volume and supply, Hims & Hers CEO Andrew Dudum, via statement


![Trump’s Shocking Overhaul:10,000 Health Workers Fired, Unions Stripped of Power-What It Means for US [Video]](https://video.newsserve.net/300/v/20250401/1406815244-Trump-Shocking-Overhaul-10-000-Health-Workers.jpg)